Musiyano wakakosha pakati pe nitrate ne nitrite ndewekuti nitrate ine maatomu matatu eokisijeni akasungirirwa kuatomu renitrogen nepo nitrite ine maatomu maviri eokisijeni akasungirirwa kuatomu renitrogen.
Zvose nitrate uye nitrite inorganic anions ine nitrogen uye maatomu eokisijeni.Maanion ose aya ane -1 magetsi.Zvinonyanya kuitika seanion yemakomisheni emunyu.Pane misiyano pakati pe nitrate uye nitrite;tichakurukura kusiyana ikoko munyaya ino.
Chii chinonzi Nitrate?
Nitrate inorganic anion ine makemikari formula NO3-.I polyatomic anion ine maatomu mana;atomu imwe yenitrogen uye matatu maatomu eokisijeni.Iyo anion ine -1 yakazara mutengo.Huremu hwehuremu hweiyo anion i62 g/mol.Uyezve, iyi anion inotorwa kubva kune yayo conjugate acid;nitric acid kana HNO3.Mune mamwe mazwi, nitrate ndiyo conjugate base ye nitric acid.
Muchidimbu, nitrate ion ine atomu imwe yenitrogen pakati inosunga nemaatomu matatu eokisijeni kuburikidza nekubatana kwemakemikari bonding.Paunenge uchitarisa chimiro chemakemikari yeanion iyi, ine matatu akafanana NO bonds (maererano neresonance structures yeanion).Nekudaro, iyo geometry yemorekuru ndeye trigonal planar.Atomu imwe neimwe yeokisijeni inotakura − 2⁄3 chaji, inopa kuzara kweanion se -1.
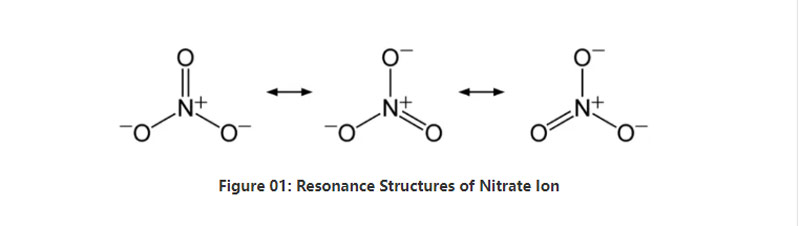
Pakumanikidza kwakajairwa uye tembiricha, anenge ese masanganiswa emunyu ane iyi anion anonyungudika mumvura.Tinogona kuwana inowanzoitika munyu yenitrate pasi pano sedhipoziti;nitratine deposits.Inonyanya kuve ine sodium nitrate.Uyezve, mabhakitiriya ari nitrifying anogona kugadzira nitrate ion.Imwe yemashandisirwo makuru emunyu wenitrate mukugadzirwa kwefetereza.Uyezve, inobatsira seanoxidizing agent mune zvinoputika.
Chii chinonzi Nitrite?
Nitrite inorganic munyu ine makemikari formula NO2–.Iyi anion anion inoenzanirana, uye ine atomu imwe yenitrogen yakasungirirwa kumaatomu maviri eokisijeni ane maviri akafanana NO covalent chemical bond.Saka, atomu yenitrogen iri pakati pemorekuru.Iyo anion ine -1 yakazara mutengo.
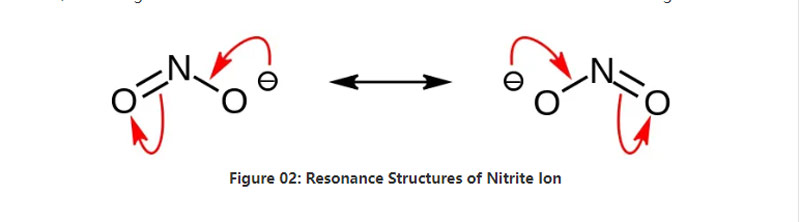
Iyo molar mass yeanion i46.01 g / mol.Zvakare, iyi anion inotorwa kubva kune nitrous acid kana HNO2.Nokudaro, ndiyo conjugate base ye nitrous acid.Naizvozvo, isu tinokwanisa kugadzira nitrite munyu muindasitiri kuburikidza nekupfuudza nitrous fumes mune aqueous sodium hydroxide solution.Uyezve, izvi zvinogadzira sodium nitrite iyo isu tinogona kuchenesa kuburikidza nerecrystallization.Uyezve, nitrite munyu senge sodium nitrite inobatsira mukuchengetedza chikafu nekuti inogona kudzivirira chikafu kubva mukukura kwehutachiona.
Ndeupi Musiyano Pakati peNitrate neNitrite?
Nitrate inorganic anion ine kemikari formula NO3– nepo Nitrite iri inorganic munyu ine kemikari formula NO2–.Nokudaro, musiyano mukuru pakati pe nitrate ne nitrite uripo pamakemikari anoumbwa emaanioni maviri.Ndiko kuti;musiyano mukuru uripo pakati pe nitrate ne nitrite ndewekuti nitrate ine maatomu matatu eokisijeni akasungirirwa kuatomu renitrogen nepo nitrite ine maatomu maviri eokisijeni akasungirirwa kuatomu renitrogen.Uyezve, nitrate ion inotorwa kubva kune yayo conjugate acid;iyo nitric acid, nepo nitrite ion inotorwa kubva ku nitrous acid.Semumwe mutsauko wakakosha pakati pe nitrate uye nitrite ions, tinogona kutaura kuti nitrate ioxidizing agent nekuti inogona kuderedzwa chete asi nitrite inogona kuita seyose oxidizing uye inoderedza mumiriri.
Nguva yekutumira: May-16-2022





